Impressive detection of very low concentrations of the neurotransmitter dopamine have just been demonstrated in research coming from the Wang and Tu research groups at the Department of Bioscience and Biotechnology at the National Taiwan Ocean University and the Institute of Chemistry at the Academia Sinica, respectively, in Taiwan. The work was recently published as an on-line article in ACS Sensors.
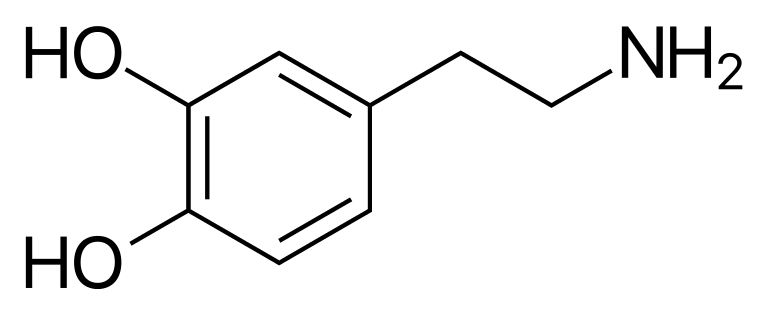
Dopamine, shown at right for like-minded chemistry nerds, is a small molecule that acts as a neurotransmitter in the brain and as a hormone with different functions elsewhere in the body including blood vessels, kidneys, the pancreas and the immune system. The ability to detect dopamine is of great interest, since abnormal dopamine levels are indicative of neurological conditions such as depression, schizophrenia, attention deficit hyperactivity disorder (ADHD) and Parkinson’s disease.
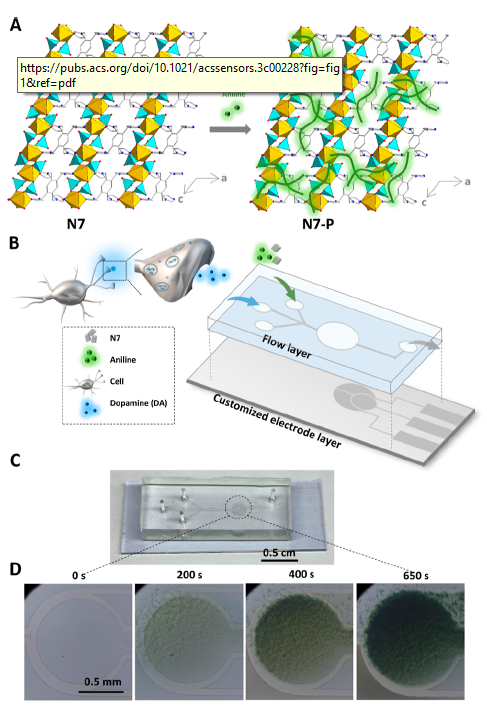
The researchers created a microfluidic device to act as the dopamine biosensor. The glass base layer had an indium tin oxide (ITO) coating patterned with a CO2 laser to form the 3-electrodes used for electrochemical detection. The fluidic cover was made by soft lithography: cast moulding of PDMS over a photolithographically patterned SU-8 master. An additional novel coating was applied to the working electrode to afford the selective sensing of dopamine. The coating, “N7-P”, was electro-polymerised via cyclic voltammetry from a solution of an indium phosphate-organic linker hybrid and analine. The graphic at right from their paper shows a) the structure of the yellow/cyan InPO4/organic linker crystalline structure (left) with added green polyaniline (right), b) electrode base (grey) and fluidic cover (blue), together with N7-P coating materials input well (top) and neuroblastoma cell/dopamine input well (middle), c) photo of assembled chip, and d) microscope imagery of working electrode during coating process of the N7-P.
Performance demonstrated by the groups’ microfluidic dopamine sensors was impressive in terms of the low detection limits and dynamic ranges seen in static and flowing configurations. With no flow, the linear range was 1 pM to 10 nM, while under flow (optimised at 50 µL/h), the range was 1 aM to 100 fM, with an LoD of 0.183 aM. Unfortunately, the authors did not disclose the dimensions of the device’s fluidic channels and detection chamber, which become significant when low concentration levels approach the need for single molecule detection, as in this case.
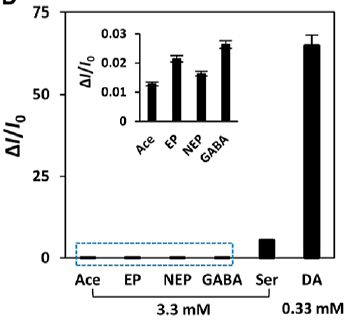
Also important was the excellent selectivity shown by the devices for sensing dopamine in the presence of five other neurotransmitters with similar structures — acetylcholine, epinephrine, norepinephrine, gamma-aminotbutyric acid and serotonin. A plot showing the amperometric response of the sensor to dopamine (DA) versus these other five potential interferents at 10-fold concentrations (labelled Ace, EP, NEP, GABA and Ser, respectively) is shown at right.
In their experiments looking at biologically produced dopamine from rat cells (> 1000 cells/mL), the concentrations measured by the microfluidic biosensor tracked very closely with the results obtained using a commercial ELISA system when larger numbers of cells were used (> 1000 cells/mL); no comparison of results could be made for smaller numbers, as the commercial system failed to generate any signal. Signal from as few as 21 cells could be sensed at dopamine concentrations of ~15 aM.
If I’ve lost you with a bunch of chemistry concentrations jargon, please accept my apologies! The salient point is, the system has exquisite sensitivity, and that can be very useful in many situations where e.g. human biopsy or research cell samples are very small, and the impact of the cells’ environment or the effectiveness of a therapeutic drug for a neurological disorder is being evaluated. Having more sensitivity is like having more horsepower: it’s (almost) always a good thing!
Another important point is that the devices are manufacturable with what look to be fairly mainstream or easily implemented microfabrication methods. They mention that the closest rival electrode coating material to afford the selectivity for dopamine are “metal organic frameworks”, or MOFs. In a table they provide (Table S2 of their supplementary information), the current biosensor’s linear range is at least 8 orders of magnitude (i.e. 80 million times) more sensitive than that seen with MOF-based electrochemical detection. It’s not clear how ease-of-microfabrication compares between MOFs and the present coating technology.
