Researchers from César Pascual García’s group at the Luxembourg Institute of Science and Technology as well as Wouter Olthuis from the MESA+ Institute at the University of Twente in the Netherlands have designed, built and characterised a microfluidic device capable of controlling pH between acidic and neutral pH values. Their work was recently published on-line as an ACS Omega pre-print article.
Why does on-chip pH control matter? Control of acidity allows many molecular states and reactions to be regulated, e.g. for the synthesis of oligonucleotides (DNA), polypeptides (proteins), saccharides (carbohydrates), all of which are key in many areas of analytical, organic and pharmaceutical chemistry. One important area is combinatorial chemistry, which allows an array of related chemical compounds to be meticulously synthesised, and then evaluated against a target molecule for a given purpose, such as therapeutic effect of a drug, or receptor affinity as a bio marker of a disease or condition to be diagnosed. Regulating a large number of such reactions in a dense matrix array aboard a microfluidic chip means that many more reaction options can be screened simultaneously with very small amounts of reagents in an automated fashion, making for more effective drugs and diagnostics with lower development costs.
Their microfluidic devices have a Si/SiO2 base with Pt working, counter and reference electrodes, SU-8 patterned fluidic channels and removable glass lid. Ti/Au electrodes were lithographically patterned using lift-off, with subsequent electrochemical deposition of Pt and functionalisation with the electro-active species 4-aminothiophenol (4-ATP) thereafter. A photo of a 4-cell arrangement of their system, pH response and reversible reaction controlling the chamber pH are shown below.
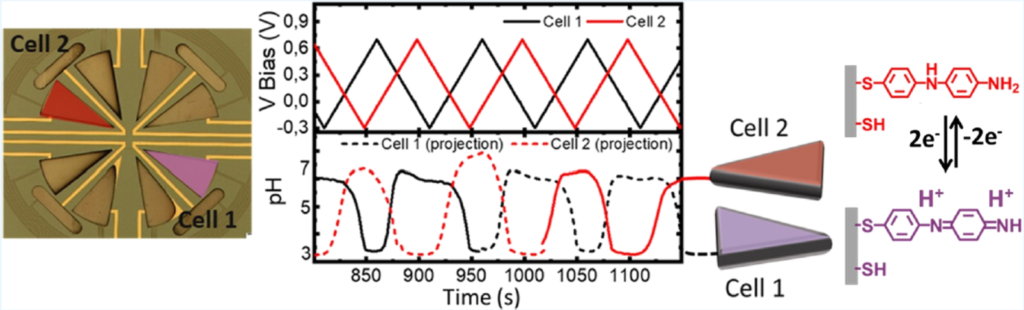
Fairly reproducible control of pH through 100+ cycles was achieved, with pH stability lasting through holding times of up to 10 minutes. pH can also be tailored to given values between pH = 3-7 ±0.4, based on the fluorescence intensity of the carboxy semi-napthorhodafluors dye used to validate performance.
The authors have taken previous work by others using photoactive compounds for pH regulation ahead a step, by removing the need for optical interrogation of the cell. This eliminates expensive optics instrumentation and alignment issues, replacing them with mass-manufacturable electrochemical control. While the current design is not configured for high density, the 2.5 nL reaction volumes are already quite small, and it is not hard to imagine a high density layout for manufacturing. Application of this technology to the development of therapeutic drug candidates, disease diagnostics and other areas seems promising.
