Xiujun Li’s group at the University of Texas at El Paso recently published an article in Analytical Chemistry that builds on the “V-chip” concept from a 2012 Nature Communications publication by the Qin group. The chip uses the position of a visible liquid in a channel to allow the user to quantitatively determine the concentration of an analyte of interest by simple visual inspection – no detector needed!
(Copyright: American Chemical Society)
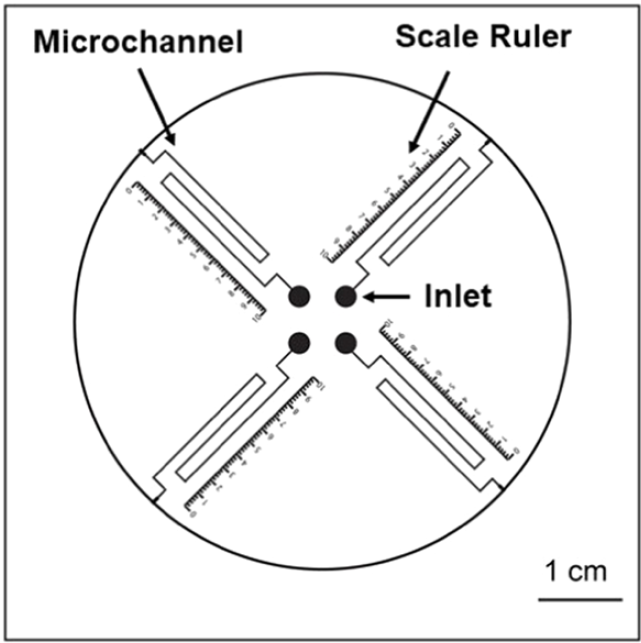
The chip (schematic at right) operates similarly to an alcohol or mercury thermometer in which the user reads the liquid meniscus level against a scale to determine the temperature. In the case of these microfluidic V-chip devices, the liquid level moves in response to the concentration of an analyte. In the seminal paper by Qin in 2012, the liquid plug was driven by gaseous products generated in a reaction with the analyte. A sandwich ELISA (enzyme-linked immunosorbent assay) reaction was used in which the unbound antibody was labelled with nanoparticles conjugated to a catalase enzyme. At the end of the ELISA reaction, the bound catalase labels reacted with preloaded hydrogen peroxide in the solution to produce oxygen gas in proportion to the amount of analyte initially present. The liquid ink was driven along connected ‘thermometer’ microfluidic channels and measured accordingly to determine the concentration of analyte. This volumetric relationship gives rise to the V in V-chip.
(Copyright: American Chemical Society)
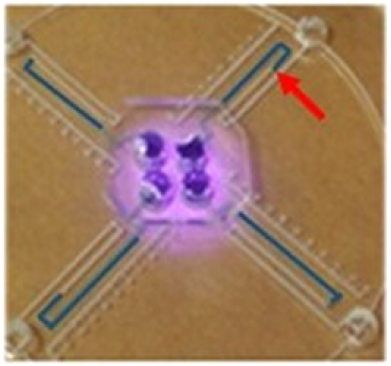
In the work of Li’s group, a sandwich ELISA is also used, but the labelling and gas generation are different, and arguably improved. Instead of generating gas enzymatically with catalase, it is generated photothermally with a near infrared (NIR) laser. In their work, the analyte of interest is the prostate cancer biomarker PSA (prostate-specific antigen), and the unbound antibody is labelled with an Fe3O4 nanoparticle. The nanoparticle is chemically converted to a Prussian Blue (PB) nanoparticle which is a strong NIR-absorbing photothermal agent. At the end of the ELISA reaction, an NIR laser is shone onto the sample chamber and the incident radiation is converted by the PB nanoparticles to heat and generates vapour, causing liquid to be driven along the thin radiating ‘thermometer’ channels. The extent of displacement of the liquid again depends directly on the amount of PB nanoparticle-labelled antibodies present, which is a function of the concentration of PSA to which they bind. An example with blue dye showing the liquid after ELISA reaction and photothermal interrogation is shown above right.
The V-chips in both studies offer the huge benefit of not requiring any measuring instrumentation for detection; no optical or electrochemical detector, signal processing and display required. With a properly designed microfluidic chip and calibrated scale, the results of an ELISA-based cancer biomarker assay can be read directly from the chip like a thermometer. The dramatic reduction in complexity and cost can be a huge gain in a derived product concept. In addition, Li’s work uses a photothermal heating process in lieu of the second enzymatic reaction (catalase) in the analysis chain, which both simplifies the procedure, and is likely to make it more robust, since photothermal heating is more controllable and reproducible than an enzymatic reaction. The cost for this is an added on- or off-board NIR laser and lens, both of which can be mass-fabricated cheaply. This approach potentially improves analytical performance, which could in turn be an enabling piece of the technology foundation for a related product.
