 Onur Gökçe at University of Zurich/ETH and Yuksel Temiz and Emmanuel Delamarche at IBM in Zurich, Switzerland, together with Samuel Castonguay and Thomas Gervais at École Polytechnique in Montréal, Canada recently authored a clever article in Nature that shows beautiful control of dried reagent dissolution and diffusion into the liquid buffer flowing over-top of the reagents in a microfluidic channel.
Onur Gökçe at University of Zurich/ETH and Yuksel Temiz and Emmanuel Delamarche at IBM in Zurich, Switzerland, together with Samuel Castonguay and Thomas Gervais at École Polytechnique in Montréal, Canada recently authored a clever article in Nature that shows beautiful control of dried reagent dissolution and diffusion into the liquid buffer flowing over-top of the reagents in a microfluidic channel.
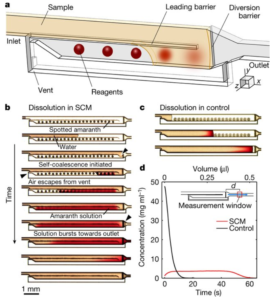
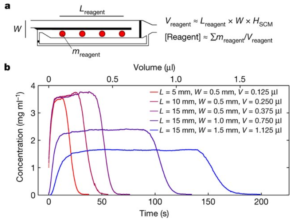
The authors demonstrated that, by including a partial-height longitudinal wall to act as a “capillary pinning line”, liquid flows through an empty channel and back along the adjoining channel with spotted reagents to create zones of dissolved reagents – a process they term ‘self-coalescing flow’ or SCM. This is in contrast with the frontal accumulation of reagents spotted conventionally along a single channel. Further, by adjusting channel dimension, the concentration profiles could easily be manipulated to produce short zones of high-concentration or longer zones of lower concentration, with fairly homogeneous concentration profiles in all cases. Several additional examples showed the ability to either segregate or coalesce such zones for a given reagent, mix and react with downstream reagents, etc.
 From the perspective of microfluidic product development, this development is very interesting for several reasons. First, it provides this control of reagents in solution with very simple channel structures that can be fabricated existing standard methods for any substrate material (plastic, glass, silicon). Secondly, it may obviate the need for more complex channel networks and associated pumps and valves required to physically segregate parallel reactions of a single analyte with several target reagents for multiplexed analyses, common in many microfluidic products currently. This could reduce the size, complexity and cost of the chip consumable, making the price and profitability of such products much more attractive.
From the perspective of microfluidic product development, this development is very interesting for several reasons. First, it provides this control of reagents in solution with very simple channel structures that can be fabricated existing standard methods for any substrate material (plastic, glass, silicon). Secondly, it may obviate the need for more complex channel networks and associated pumps and valves required to physically segregate parallel reactions of a single analyte with several target reagents for multiplexed analyses, common in many microfluidic products currently. This could reduce the size, complexity and cost of the chip consumable, making the price and profitability of such products much more attractive.
