New work from Patrick Doyle’s group at MIT, just published in ACS’ Applied Polymer Materials and highlighted in MIT News, shows the simple microfluidic manufacture of hydrogel particles that can be used to remove hydrophobic (non-polar, water ‘fearing’) micropollutants from water. Their hydrogel ‘filtres’ show enhanced performance compared to activated carbon (AC); in addition, and critically, they can easily be regenerated.
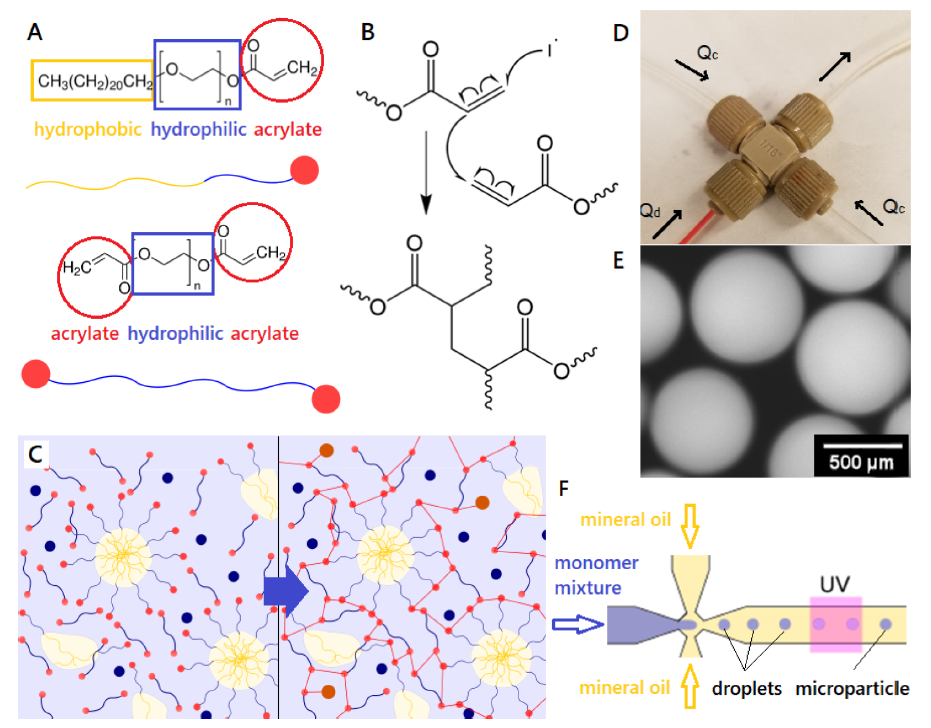
The hydrogel particles are made from acrylate-based micelles that are covalently bound into a cross-linked acrylate gel. The diagram above from their paper illustrates the nature of the micelle monomers and cross-linkers (A); the polymerisation reaction (B – I’ll pretend I understand exactly what’s going on, there …;-); the solution (left) before polymerisation, with free micelles spontaneously formed, and (right) after (UV) polymerisation, with the micelles fixed in the gel polymer (C); the microfluidic cross used for droplet/particle formation (D); a micrograph of the 500 µm gel particles (E); and a diagram showing the formation of the droplets in the microfluidic reactor that polymerise under UV light to form the gel particles.
The microfluidic reactor in this case is simply made from off-the-shelf components: a microcross such as from Idex (150 µm through-hole) and 1/16″ OD tubing. For R&D, this is an ideal set-up: fairly cheap with parts delivered in days and set up in minutes. For manufacturing, it’s only a small leap to replicate this structure in polymer or glass and set multiple microfluidic synthesis networks up together in parallel to reduce the cost contribution of the microfluidic reactor.
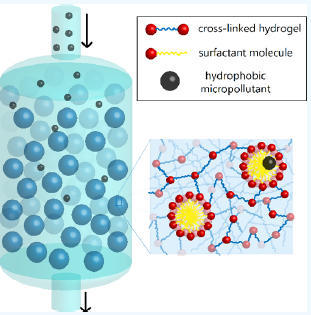
To remove the pollutants from a water sample, water is passed through a column filled with this hydrogel, and the hydrophobic pollutants partition into the hydrophobic micelle centres, analogous to reverse-phase chromatography, with the pollutants being highly retained in the stationary phase. Once the hydrogel is saturated with pollutants, it can be regenerated with an ethanol flush, purging the column of bound pollutants; the authors claim it will maintain performance over years of such regenerative cycles. Different micelle monomer hydrophobic tails can be used to optimise the affinity and partitioning coefficient for a variety of pollutant targets.
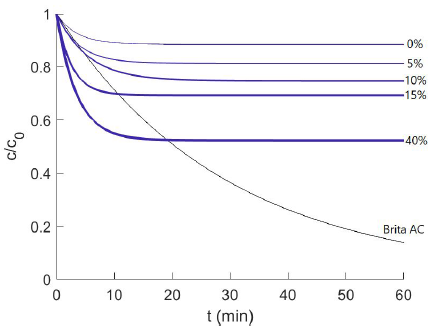
The performance of the hydrogels stacks up very nicely against activated carbon filtres which are the current gold standard in many applications. Doyle’s group measured the performance of a variety of different micelle compounds in their hydrogels against each other and activated carbon for their micropollutant model compound, 2-napthol (used in the manufacture of dyes, fungicides, insecticides, pharmaceuticals and perfumes; carries the H400 classification of “very toxic to aquatic life”). The graph at right plots pollutant removal over time for a particular micelle formulation, F127DA. The ordinate is c/c(o), or the concentration of pollutant left in the test solution vs. initial concentration; the faster and greater this drops, the better the performance. The four different exponential decay curves (blue) correspond to different quantities of micelle surfactants bound in the hydrogel: 0 to 40% surfactant concentration in the gel; the grey curve is for Brita activated carbon. The plot shows that higher percent quantities of micelles bound in the hydrogel lead to pollutant removal in greater quantities and at faster rates. On time scales under 10 minutes, typical for water treatment contact times, both the 15 and 40% F127DA formulations handily outperform the activated carbon, although longer timescales favour the AC.
The authors highlight the fact that their hydrogels can be regenerated with ethanol, using between one thousandth to one billionth the volume of EtOH vs. water treated, and show good performance for cycles subsequent to regeneration. They also note the very energy-intensive process needed for regenerating AC filtres. While it seems they have effectively shown good performance from their hydrogels for both filtration and re-usability, what is missing in the comparison, in my view, is a clearer contrast of economic and environmental drivers. For example, potential costs/year for filtre replacement, ethanol/energy consumption, releases to the environment, etc. That is arguably the product developer’s job, and perhaps this paper’s performance will pique the interest of some entrepreneurs, hopefully with well-heeled partners.
