Impressive research coming out of the Revzin group at the Mayo Clinic’s Department of Physiology and Biomedical Engineering shows an improved approach to first culture and then evaluate cell responses to chemotherapy treatments using polydimethyl siloxane (PDMS) microfluidic devices cast from silicon masters. The research was published in Microsystems & Nanoengineering in October (DOI: 10.1038/s41378-020-00201-6).
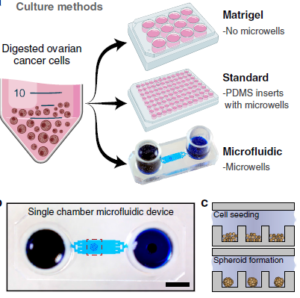 Ovarian cancer often has a very poor prognosis when detected due in large part to the inaccessibility of the ovaries for many diagnostic methods, and the late-stage in which diagnoses are usually made. As a result, there is a strong focus on improved therapeutic strategies to best use the brief window available for treatment of the advanced disease. One such strategy is to use ‘patient-derived xenografts’ (PDXs) where tumour tissue cells are implanted in a mouse to allow the cancer to be monitored in parallel to that of the patient. While valuable to determine the effectiveness of various chemotherapies, PDX requires research animals, experienced animal researchers, high costs and long timelines. As a result, in vitro ‘organoid’ or ‘spheroid’ 3-D cancer cultures are being explored on 96-well microtiter plates, Matrigel plates and other array platforms to arrive at suitable replacement environments. As comparison standards for their microfluidic design, the authors used Matrigel and microtiter plates approaches, with the latter having PDMS inserts with microwells identical in size to the microfluidic counterpart. A graphic depicting the three approaches is shown above at right.
Ovarian cancer often has a very poor prognosis when detected due in large part to the inaccessibility of the ovaries for many diagnostic methods, and the late-stage in which diagnoses are usually made. As a result, there is a strong focus on improved therapeutic strategies to best use the brief window available for treatment of the advanced disease. One such strategy is to use ‘patient-derived xenografts’ (PDXs) where tumour tissue cells are implanted in a mouse to allow the cancer to be monitored in parallel to that of the patient. While valuable to determine the effectiveness of various chemotherapies, PDX requires research animals, experienced animal researchers, high costs and long timelines. As a result, in vitro ‘organoid’ or ‘spheroid’ 3-D cancer cultures are being explored on 96-well microtiter plates, Matrigel plates and other array platforms to arrive at suitable replacement environments. As comparison standards for their microfluidic design, the authors used Matrigel and microtiter plates approaches, with the latter having PDMS inserts with microwells identical in size to the microfluidic counterpart. A graphic depicting the three approaches is shown above at right.
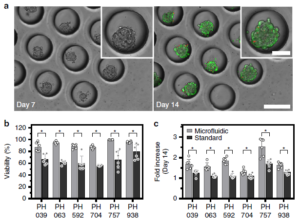 A first advantage of their microfluidic platform is that it can easily make use of the miniscule amounts of cells afforded by a fine needle biopsy sample. Secondly, the on-chip incubation environment appears to be superior to that of standard microtiter plates when using identical media and culturing methods. After 14 days, 0n-chip viability was typically ~90% vs. ~60% & ~80% for standard microtiter and Matrigel plates, respectively; growth of the spheroid cluster of cancer cells was also significantly better using the chip platform. Figure at right shows micrographs of the spheroid cell clusters in the microfluidic wells (a), and a comparison of viability (b) and spheroid size growth multiple (c) over 2 weeks. Lastly, a multiplexed design shown below
A first advantage of their microfluidic platform is that it can easily make use of the miniscule amounts of cells afforded by a fine needle biopsy sample. Secondly, the on-chip incubation environment appears to be superior to that of standard microtiter plates when using identical media and culturing methods. After 14 days, 0n-chip viability was typically ~90% vs. ~60% & ~80% for standard microtiter and Matrigel plates, respectively; growth of the spheroid cluster of cancer cells was also significantly better using the chip platform. Figure at right shows micrographs of the spheroid cell clusters in the microfluidic wells (a), and a comparison of viability (b) and spheroid size growth multiple (c) over 2 weeks. Lastly, a multiplexed design shown below  at right was created to allow serial and parallel perfusion across eight chambers. Chambers are filled serially from left as in (a), and in parallel via discrete inputs as in (b); fluidic control is achieved by opening and closing red valves surrounding each chamber. This device was used to determine the chemotherapy agent doxorubicin’s IC50 (50% inhibitory concentration) for two types of cancer cells via viability curves drawn from parallel injections of agent concentrations ranging from 10 nM to 100 µM. The injected amount for the multiplexed study was ~100,000 cells, stated as the minimum amount obtained from a fine needle aspirate (biopsy).
at right was created to allow serial and parallel perfusion across eight chambers. Chambers are filled serially from left as in (a), and in parallel via discrete inputs as in (b); fluidic control is achieved by opening and closing red valves surrounding each chamber. This device was used to determine the chemotherapy agent doxorubicin’s IC50 (50% inhibitory concentration) for two types of cancer cells via viability curves drawn from parallel injections of agent concentrations ranging from 10 nM to 100 µM. The injected amount for the multiplexed study was ~100,000 cells, stated as the minimum amount obtained from a fine needle aspirate (biopsy).
Application of this microfluidic analysis approach to very small masses of biopsy, or other, samples is compelling as demonstrated in the case of ovarian cancer. It may be that many disease biopsies from other organs or perhaps forensic or other bio-samples could also benefit from the efficient approach.
