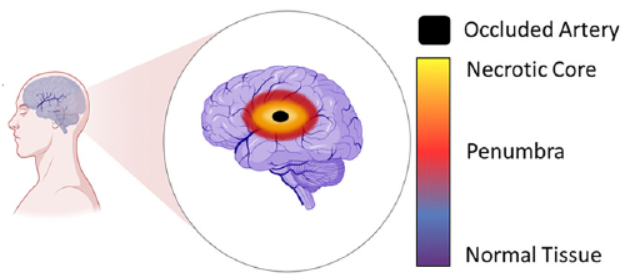
New research published in Lab on a Chip (September 21st) by researchers from the Ayuso lab (University of Wisconsin) and the Euan MacDonald Centre (University of Edinburgh) is an elegant example of a microfluidic organ-on-a-chip device. In this case, the organ is the brain, and the target disease is stroke. Stroke, or “brain attack”, is the brain equivalent of a heart attack. The majority of strokes are ischemic, typically caused by blood vessel blockage that leads to cell death in the brain tissue area served by that vessel.
The two research groups showed impressive results using rat astrocyte brain cells (which are a kind of “glia” — cells that support the brain’s neurons) to model the damage resulting from ischemic strokes, the response to reperfusion of nutrients to the deprived cells, and the viability of tissue grafting in an area damaged by stroke.
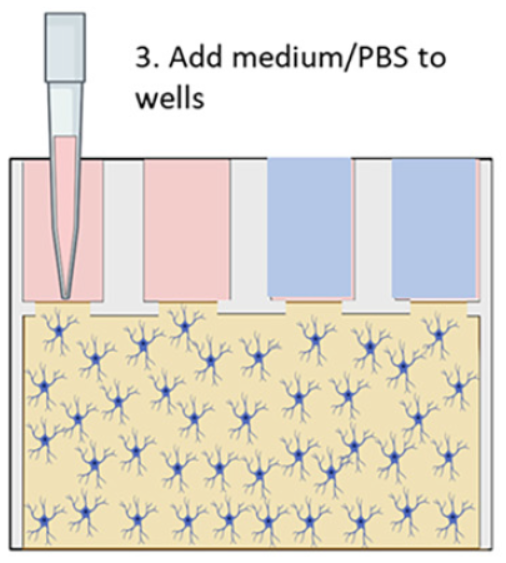
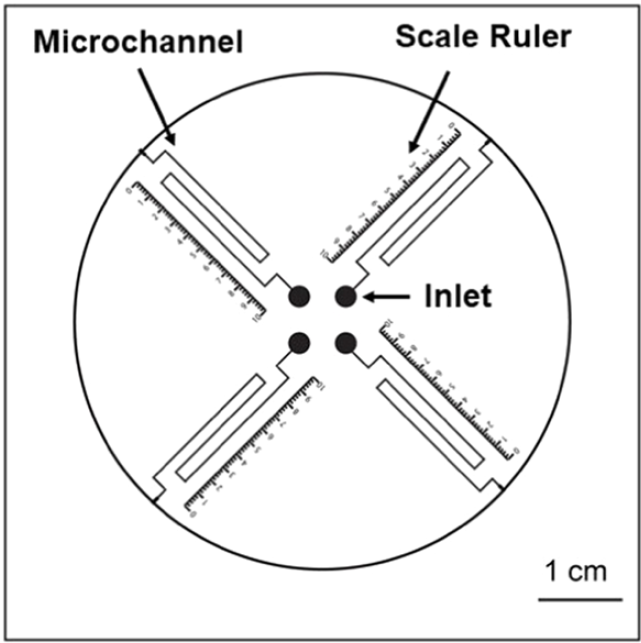
Their microfluidic brain-on-a-chip device was made from a 384-well microtiter plate with various bottom chamber configurations machined out via CNC milling, and sealed with a polystyrene laminate using double-sided tape or solvent-assisted bonding. The chambers were filled with a collagen gel/astrocyte suspension (a proxy to mimic brain tissue) through the upper well openings, and then covered by filling the wells with either a nourishing medium, to simulate normal conditions, or a simple phosphate/borate pH buffer solution (PBS) lacking nutrients, to simulate deprivation during a stroke; see Figure 2 above.
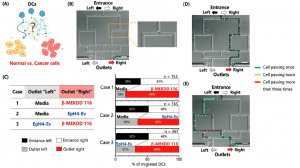
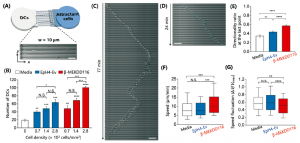
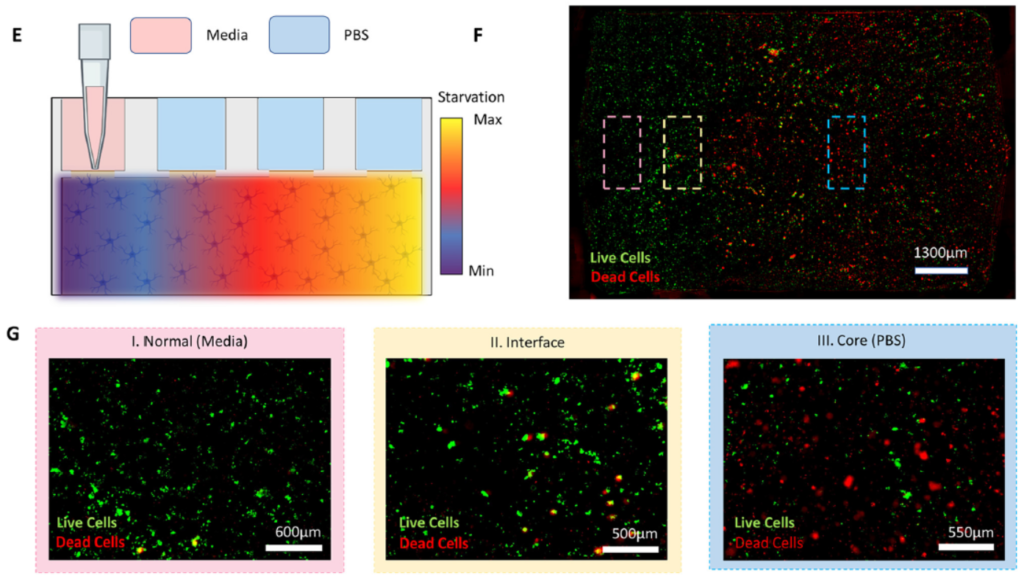
Experiments were performed to show that their simulated normal and stroke conditions over different periods of time were reflected in terms of cell viability. In addition, the extent to which cells deprived of nutrients and oxygen during a simulated stroke were able to recover when replenished with nourishing medium was explored. The schematic shown below at left in Figure 3 illustrates a simple stroke simulation experiment (E), while the microscope image at right (F) and below (G) show the resultant effects on the astrocyte cells. Live cells are stained with with a green fluorescent dye (Cell Tracker Green), and dead cells with a red dye (propidium iodide). Magnified views of the cells directly beneath, nearby, or far from the nourishing medium are also shown (G); the further from the medium, the higher the proportion of dead cells.
Adding different labelling and assaying approaches to the microscopy techniques above, they were able to model several other key stroke features. They demonstrated that reperfusion of the damaged pseudo-tissue area with nutrients from the medium after the simulated stroke did not reverse the damage done, replicating what has been observed clinically. Stroke damage was also evident in the deprived and reperfused cells vs. healthy cells by the dysregulation of certain genes critical to governing glucose metabolism; these experiments were performed by quantifying up- and down-regulation of genes using RT-qPCR on RNA obtained by bead extraction from the astrocyte-gel matrix at different stages of the experiment.
Lastly, and perhaps most excitingly, the researchers were able to show the brain-on-a-chip’s ability to potentially evaluate therapeutic treatment candidates. They punched a hole in the astrocyte gel matrix, added a suspension of macrophages (aka white blood cells; they are said to be “key players in the inflammatory response that follows stroke damage”) to the space, and imaged their movement over time with fluorescence and confocal microcroscopy. A schematic of the experiment is shown in Figure 4 below. Significant penetration into the surrounding pseudo-tissue was seen at 24h vs. 30 min.
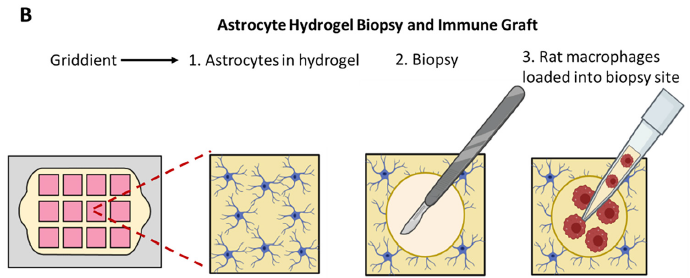
The importance of these results is significant. The research team has shown that it can monitor the health and location of astrocyte cells with a number of analytical techniques including live- and dead-cell fluorescent tagging, and monitoring of gene regulation. It has also demonstrated that their attempt at tissue grafting showed successful cell penetration. This at least means that their brain-on-a-chip device should be tested more rigorously to explore its capabilities on several fronts — for example, monitoring movement and viability of different cells within a variety of tissues/proxies, evaluating the effectiveness of emerging, unproven stroke therapeutic treatments, etc. Furthering this research could provide invaluable insight into stroke tissue damage and regeneration techniques. Also, there is no reason to think that a similar approach could not be taken with other tissue models to characterise diseases and conditions, and evaluate therapies.

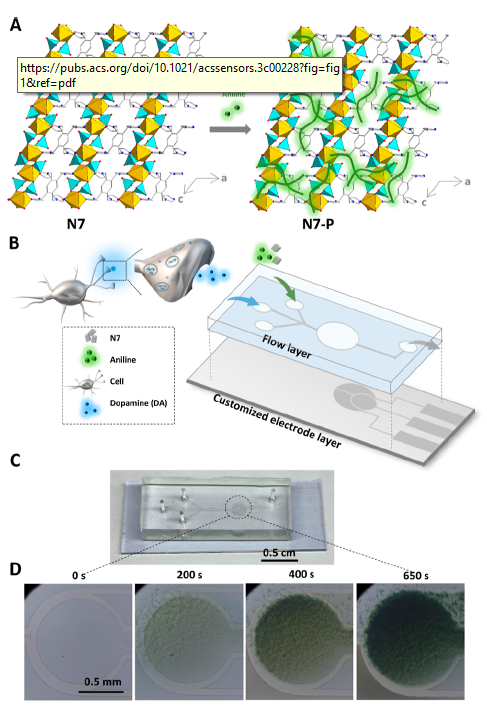




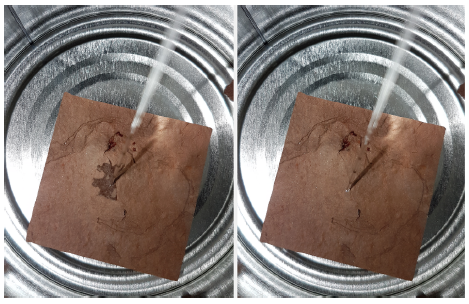
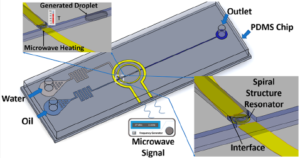
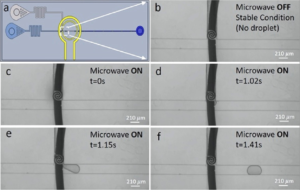 Time-lapsed photographs of droplet generation of water (vertical channel) in carrier oil (horizontal channel) using their system are shown at right below; the curved black line is the electrode under the vertical channel. The research showed a relationship between applied microwave power and generation time, but with droplet sizes remaining at a constant ~1.8 nL in their 40 µm deep x 210 µm wide channels.
Time-lapsed photographs of droplet generation of water (vertical channel) in carrier oil (horizontal channel) using their system are shown at right below; the curved black line is the electrode under the vertical channel. The research showed a relationship between applied microwave power and generation time, but with droplet sizes remaining at a constant ~1.8 nL in their 40 µm deep x 210 µm wide channels.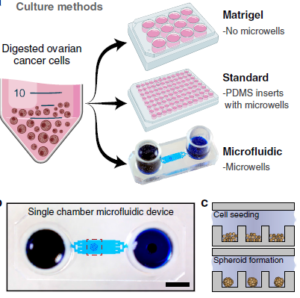
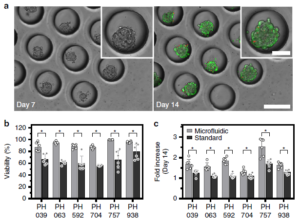 A first advantage of their microfluidic platform is that it can easily make use of the miniscule amounts of cells afforded by a fine needle biopsy sample. Secondly, the on-chip incubation environment appears to be superior to that of standard microtiter plates when using identical media and culturing methods. After 14 days, 0n-chip viability was typically ~90% vs. ~60% & ~80% for standard microtiter and Matrigel plates, respectively; growth of the spheroid cluster of cancer cells was also significantly better using the chip platform. Figure at right shows micrographs of the spheroid cell clusters in the microfluidic wells (a), and a comparison of viability (b) and spheroid size growth multiple (c) over 2 weeks. Lastly, a multiplexed design shown below
A first advantage of their microfluidic platform is that it can easily make use of the miniscule amounts of cells afforded by a fine needle biopsy sample. Secondly, the on-chip incubation environment appears to be superior to that of standard microtiter plates when using identical media and culturing methods. After 14 days, 0n-chip viability was typically ~90% vs. ~60% & ~80% for standard microtiter and Matrigel plates, respectively; growth of the spheroid cluster of cancer cells was also significantly better using the chip platform. Figure at right shows micrographs of the spheroid cell clusters in the microfluidic wells (a), and a comparison of viability (b) and spheroid size growth multiple (c) over 2 weeks. Lastly, a multiplexed design shown below  at right was created to allow serial and parallel perfusion across eight chambers. Chambers are filled serially from left as in (a), and in parallel via discrete inputs as in (b); fluidic control is achieved by opening and closing red valves surrounding each chamber. This device was used to determine the chemotherapy agent doxorubicin’s IC50 (50% inhibitory concentration) for two types of cancer cells via viability curves drawn from parallel injections of agent concentrations ranging from 10 nM to 100 µM. The injected amount for the multiplexed study was ~100,000 cells, stated as the minimum amount obtained from a fine needle aspirate (biopsy).
at right was created to allow serial and parallel perfusion across eight chambers. Chambers are filled serially from left as in (a), and in parallel via discrete inputs as in (b); fluidic control is achieved by opening and closing red valves surrounding each chamber. This device was used to determine the chemotherapy agent doxorubicin’s IC50 (50% inhibitory concentration) for two types of cancer cells via viability curves drawn from parallel injections of agent concentrations ranging from 10 nM to 100 µM. The injected amount for the multiplexed study was ~100,000 cells, stated as the minimum amount obtained from a fine needle aspirate (biopsy).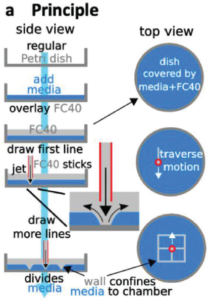
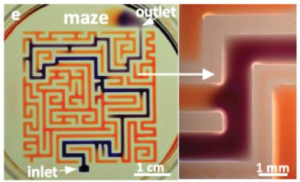 The authors have done a nice job of characterising the various parameters relevant to creating walls or features from the fluorocarbon, such as jet nozzle height, diameter, flow rate, lateral speed, wall thickness and channel widths. The ability to pipet into enclosed chamber arrays was also explored in terms of volume ranges and resistance to cross-contamination. Preliminary cloning studies with different cells suggested comparable cloning efficiency vs. normal Petri dishes without fluorocarbon structures.
The authors have done a nice job of characterising the various parameters relevant to creating walls or features from the fluorocarbon, such as jet nozzle height, diameter, flow rate, lateral speed, wall thickness and channel widths. The ability to pipet into enclosed chamber arrays was also explored in terms of volume ranges and resistance to cross-contamination. Preliminary cloning studies with different cells suggested comparable cloning efficiency vs. normal Petri dishes without fluorocarbon structures.
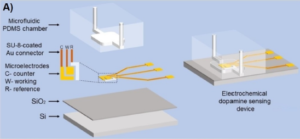 The microfluidic devices were fairly simple in structure, with a three-electrode configuration used for the amperometric detection patterned on the oxide layer of a silicon substrate, and a single channel/chamber fluidic structure fabricated in PDMS that was bonded to the silicon baseplate.
The microfluidic devices were fairly simple in structure, with a three-electrode configuration used for the amperometric detection patterned on the oxide layer of a silicon substrate, and a single channel/chamber fluidic structure fabricated in PDMS that was bonded to the silicon baseplate.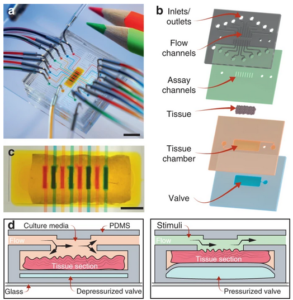
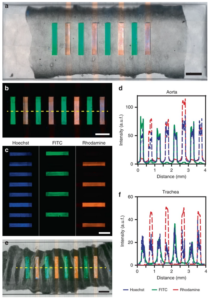 tissue is pressed against the lid to seal the microfluidic channels and allow the simultaneous introduction of discrete drug or tissue culture solutions through each of the eight channels. Characterisation studies shown in the lower image (©Nature – Microsystems & Nanoengineering) demonstrate good permanent sealing and no cross-talk between channels. Also, the continuous periperfusion of media around the sample increased tissue cell viability over the first several hours in the microfluidic device vs. in a petri dish.
tissue is pressed against the lid to seal the microfluidic channels and allow the simultaneous introduction of discrete drug or tissue culture solutions through each of the eight channels. Characterisation studies shown in the lower image (©Nature – Microsystems & Nanoengineering) demonstrate good permanent sealing and no cross-talk between channels. Also, the continuous periperfusion of media around the sample increased tissue cell viability over the first several hours in the microfluidic device vs. in a petri dish.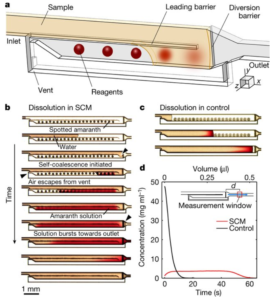
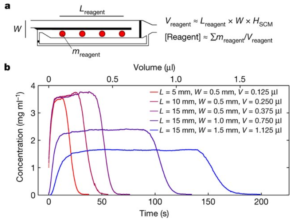
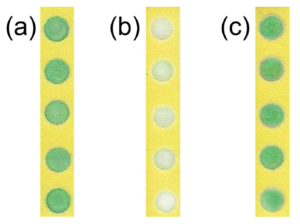
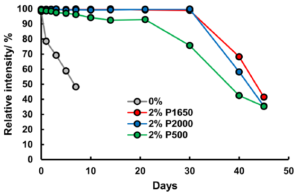 To stabilise the hydrogen peroxide, they added PVA to the hydrogen peroxide solutions prior to addition to the PAD, and found dramatic improvements in hydrogen peroxide stability. The extent of the improvement varying with PVA concentration and chain length, as well as storage temperature. 2% solutions of PVA with a length of ~1650 monomers extended the life of the reaction at ~100 intensity to 10 days with storage at room temperature, while refrigeration at 4°C extended that to 30 days for PVA lengths of ~1650 or 2000 monomers. The PVA is thought to protect the hydrogen peroxide by forming a liquid- and air-tight barrier, thus preventing attack by hydroxide anion that catalyses the degradation.
To stabilise the hydrogen peroxide, they added PVA to the hydrogen peroxide solutions prior to addition to the PAD, and found dramatic improvements in hydrogen peroxide stability. The extent of the improvement varying with PVA concentration and chain length, as well as storage temperature. 2% solutions of PVA with a length of ~1650 monomers extended the life of the reaction at ~100 intensity to 10 days with storage at room temperature, while refrigeration at 4°C extended that to 30 days for PVA lengths of ~1650 or 2000 monomers. The PVA is thought to protect the hydrogen peroxide by forming a liquid- and air-tight barrier, thus preventing attack by hydroxide anion that catalyses the degradation.
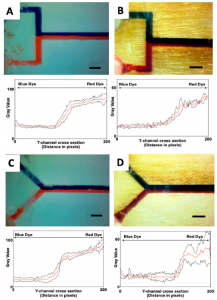 Runs with blue and red food dye mixed at Y- and T-intersection mixers were performed and imaged to compare performance in a simple flow experiment. The figure at right shows comparable performance (PMMA in left column, wood in right column), though for both geometries, the wood chips do not provide an even amount of the two dyes (less red dye), and the signal is noisier, as shown by the black standard deviation lines above and below the red average signal lines. No discussion was found concerning these two discrepancies.
Runs with blue and red food dye mixed at Y- and T-intersection mixers were performed and imaged to compare performance in a simple flow experiment. The figure at right shows comparable performance (PMMA in left column, wood in right column), though for both geometries, the wood chips do not provide an even amount of the two dyes (less red dye), and the signal is noisier, as shown by the black standard deviation lines above and below the red average signal lines. No discussion was found concerning these two discrepancies.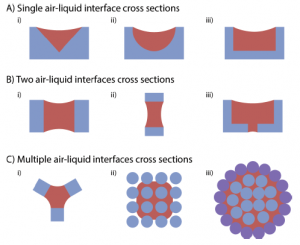
 The authors review a number of different channel geometries and fibre bundle configurations that can be used to promote open capillary flow. The helpfully provide the equations that use channel geometry and contact angle to determine whether capillary flow is energetically favourable. This is done for both conventional monolithic (single material) channels, as well as composite material channels.
The authors review a number of different channel geometries and fibre bundle configurations that can be used to promote open capillary flow. The helpfully provide the equations that use channel geometry and contact angle to determine whether capillary flow is energetically favourable. This is done for both conventional monolithic (single material) channels, as well as composite material channels.